Consider the titration curves (labeled a and b) for two weak acids, both titrated with 0.100 M NaOH.
(ii) Which acid has the larger Ka?

 Verified step by step guidance
Verified step by step guidance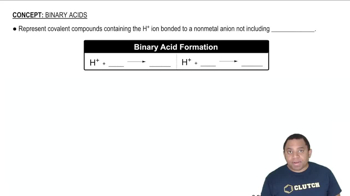
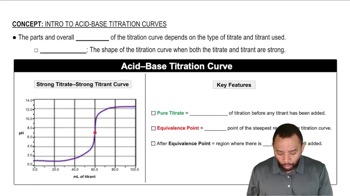

Consider the titration curves (labeled a and b) for two weak acids, both titrated with 0.100 M NaOH.
(ii) Which acid has the larger Ka?
Consider the titration curves (labeled a and b) for two weak bases, both titrated with 0.100 M HCl. (a)
(b)
(ii) Which base has the larger Kb?
A 0.229-g sample of an unknown monoprotic acid is titrated with 0.112 M NaOH. The resulting titration curve is shown here. Determine the molar mass and pKa of the acid.
A 20.0-mL sample of 0.115 M sulfurous acid (H2SO3) solution is titrated with 0.1014 M KOH. At what added volume of base solution does each equivalence point occur?
Methyl red has a pKa of 5.0 and is red in its acid form and yellow in its basic form. If several drops of this indicator are placed in a 25.0-mL sample of 0.100 M HCl, what color will the solution appear? If 0.100 M NaOH is slowly added to the HCl sample, in what pH range will the indicator change color?