Textbook Question
A formic acid solution has a pH of 3.25. Which of these substances will raise the pH of the solution upon addition? Explain your answer. a. HCl b. NaBr c. NaCHO2 d. KCl

 Verified step by step guidance
Verified step by step guidance
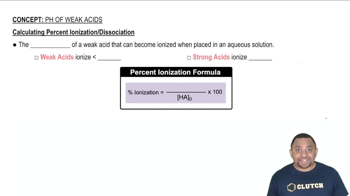


A formic acid solution has a pH of 3.25. Which of these substances will raise the pH of the solution upon addition? Explain your answer. a. HCl b. NaBr c. NaCHO2 d. KCl
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. a solution that is 0.20 M in HCHO2 and 0.15 M in NaCHO2
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. b. a solution that is 0.16 M in NH3 and 0.22 M in NH4Cl