A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. c. What is the pH after addition of 85.0 mg of NaOH?
Ch.17 - Aqueous Ionic Equilibrium

Chapter 17, Problem 49
For each solution, calculate the initial and final pH after adding 0.010 mol of HCl: a. 500.0 mL of pure water b. 500.0 mL of a buffer solution that is 0.125 M in HC2H3O2 and 0.115 M in NaC2H3O2 c. 500.0 mL of a buffer solution that is 0.155 M in C2H5NH2 and 0.145 M in C2H5NH3Cl.
 Verified step by step guidance
Verified step by step guidance1
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is neutral, below 7 indicates acidity, and above 7 indicates basicity. The pH is calculated using the formula pH = -log[H+], where [H+] is the concentration of hydrogen ions in the solution. Understanding pH is crucial for predicting how the addition of acids or bases will affect a solution.
Recommended video:
Guided course

The pH Scale
Buffer Solutions
Buffer solutions are mixtures that resist changes in pH when small amounts of acid or base are added. They typically consist of a weak acid and its conjugate base or a weak base and its conjugate acid. The Henderson-Hasselbalch equation can be used to calculate the pH of buffer solutions, which is essential for understanding how they maintain stability in pH during the addition of HCl in the given scenarios.
Recommended video:
Guided course

Buffer Solutions
Dilution and Concentration Changes
When a solute is added to a solution, the concentration of the solute changes, which can affect the pH. In the case of adding HCl to water or buffer solutions, the initial concentration of H+ ions must be considered, along with the volume of the solution. The final pH can be calculated by determining the new concentration of H+ ions after the addition of HCl, which is critical for accurately predicting the pH changes in the solutions.
Recommended video:
Guided course
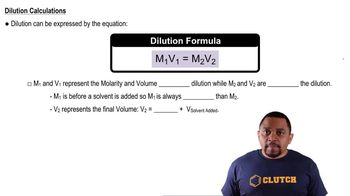
Dilution Equation
Related Practice
Textbook Question
Textbook Question
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. a. What is the initial pH of this solution?
Textbook Question
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. b. What is the pH after addition of 150.0 mg of HBr?
Textbook Question
For each solution, calculate the initial and final pH after adding 0.010 mol of NaOH. a. 250.0 mL of pure water b. 250.0 mL of a buffer solution that is 0.195 M in HCHO2 and 0.275 M in KCHO2 c. 250.0 mL of a buffer solution that is 0.255 M in CH3CH2NH2 and 0.235 M in CH3CH2NH3Cl
Textbook Question
A 100.0-mL buffer solution is 0.100 M in NH3 and 0.125 M in NH4Br. What mass of HCl can this buffer neutralize before the pH falls below 9.00?
2
views
