Calculate the molar solubility of calcium hydroxide in a solution buffered at each pH. c. pH = 9
Ch.17 - Aqueous Ionic Equilibrium

Chapter 17, Problem 100
Determine if each compound is more soluble in acidic solution than it is in pure water. Explain. a. Hg2Br2 b. Mg(OH)2 c. CaCO3 d. AgI
 Verified step by step guidance
Verified step by step guidance1
Identify the nature of each compound and its solubility in water.
Consider the effect of an acidic solution on the solubility of each compound.
For Hg_2Br_2, note that it is a salt of a weak acid (HBr) and a weak base (Hg_2^2+), and its solubility is not significantly affected by acidic solutions.
For Mg(OH)_2, recognize that it is a basic compound. In an acidic solution, the H+ ions will react with OH- ions to form water, increasing its solubility.
For CaCO_3, understand that it is a salt of a weak acid (H_2CO_3). In an acidic solution, H+ ions will react with CO_3^2- ions to form H_2CO_3, increasing its solubility.
For AgI, note that it is a salt of a weak acid (HI) and a weak base (Ag+), and its solubility is not significantly affected by acidic solutions.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility and pH
Solubility refers to the ability of a substance to dissolve in a solvent, which can be influenced by the pH of the solution. In acidic solutions, the concentration of hydrogen ions (H+) increases, which can affect the solubility of certain compounds, particularly those that can react with acids or have basic properties.
Recommended video:
Guided course
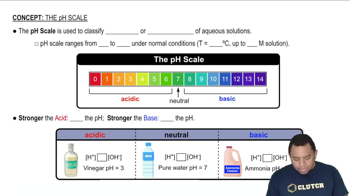
The pH Scale
Common Ion Effect
The common ion effect describes the decrease in solubility of a salt when a common ion is added to the solution. For example, adding a salt that shares an ion with the compound in question can shift the equilibrium, reducing its solubility. This concept is crucial for understanding how the presence of ions in acidic solutions can impact solubility.
Recommended video:
Guided course
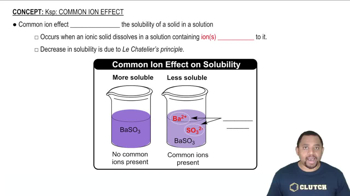
Common Ion Effect
Acid-Base Reactions
Acid-base reactions involve the transfer of protons (H+) between reactants. Some compounds, like carbonates and hydroxides, can react with acids to form soluble products. Understanding these reactions helps predict how compounds like CaCO3 and Mg(OH)2 will behave in acidic solutions, potentially increasing their solubility.
Recommended video:
Guided course
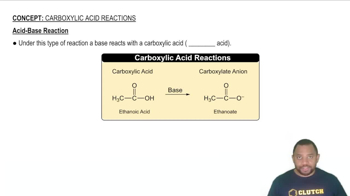
Acid-Base Reaction
Related Practice
Textbook Question
Textbook Question
Calculate the solubility (in grams per 1.00⨉102 mL of solution) of magnesium hydroxide in a solution buffered at pH = 10. How does this compare to the solubility of Mg(OH)2 in pure water?
Textbook Question
Determine if each compound is more soluble in acidic solution than it is in pure water. Explain. a. BaCO3 b. CuS c. AgCl d. PbI2
1
views
Textbook Question
A solution containing sodium fluoride is mixed with one containing calcium nitrate to form a solution that is 0.015 M in NaF and 0.010 M in Ca(NO3)2. Does a precipitate form in the mixed solution? If so, identify the precipitate.
Textbook Question
Predict whether a precipitate will form if you mix 75.0 mL of a NaOH solution with pOH = 2.58 with 125.0 mL of a 0.018 M MgCl2 solution. Identify the precipitate, if any.
