Ch.17 - Aqueous Ionic Equilibrium

Chapter 17, Problem 8
A 20.0-mL sample of a 0.125 M diprotic acid (H2A) solution is titrated with 0.1019 M KOH. The acid ionization constants for the acid are Ka1 = 5.2 * 10^-5 and Ka2 = 3.4 * 10^-10. At what added volume of base does each equivalence point occur?
 Verified step by step guidance
Verified step by step guidance1
Calculate the moles of the diprotic acid (H2A) using the initial concentration and volume: \( \text{moles of } H_2A = 0.125 \text{ M} \times 0.0200 \text{ L} \).
Determine the moles of KOH needed to reach the first equivalence point, where all \( H_2A \) is converted to \( HA^- \). This is equal to the initial moles of \( H_2A \).
Calculate the volume of KOH solution required to reach the first equivalence point using the moles of KOH and its concentration: \( \text{Volume of KOH} = \frac{\text{moles of KOH}}{0.1019 \text{ M}} \).
Determine the moles of KOH needed to reach the second equivalence point, where all \( HA^- \) is converted to \( A^{2-} \). This is twice the initial moles of \( H_2A \).
Calculate the total volume of KOH solution required to reach the second equivalence point using the moles of KOH and its concentration: \( \text{Total Volume of KOH} = \frac{\text{moles of KOH for second equivalence point}}{0.1019 \text{ M}} \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Diprotic Acids
Diprotic acids are acids that can donate two protons (H⁺ ions) per molecule in a solution. This characteristic leads to two distinct ionization steps, each with its own acid dissociation constant (Ka). Understanding the behavior of diprotic acids is crucial for determining the equivalence points during titration, as each proton donation corresponds to a different stage in the titration process.
Recommended video:
Guided course
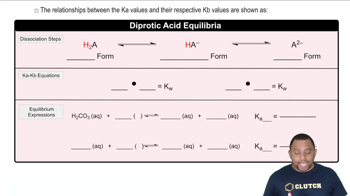
3 forms of Diprotic Acids
Titration and Equivalence Points
Titration is a quantitative analytical method used to determine the concentration of a solute in a solution. The equivalence point in a titration occurs when the amount of titrant added is stoichiometrically equivalent to the amount of substance being titrated. For diprotic acids, there are two equivalence points, corresponding to the complete neutralization of each proton, which can be calculated using the initial concentration and volume of the acid solution.
Recommended video:
Guided course
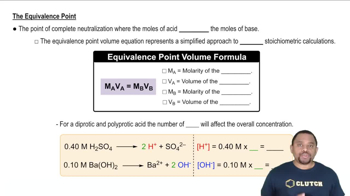
Equivalence Point in Titration
Acid-Base Neutralization Reaction
An acid-base neutralization reaction involves the reaction of an acid with a base to produce water and a salt. In this context, KOH acts as a strong base that reacts with the diprotic acid H2A. The stoichiometry of the reaction is essential for calculating the volume of base required to reach each equivalence point, as it determines how many moles of KOH are needed to neutralize the protons released by the acid.
Recommended video:
Guided course

Acid-Base Reaction
Related Practice
