Textbook Question
Caffeine (C8H10N4O2) is a weak base with a pKb of 10.4. Calculate the pH of a solution containing a caffeine concentration of 455 mg/L.
1
views

 Verified step by step guidance
Verified step by step guidance
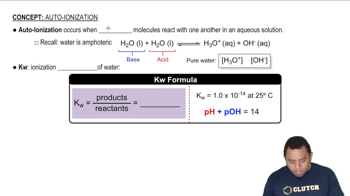
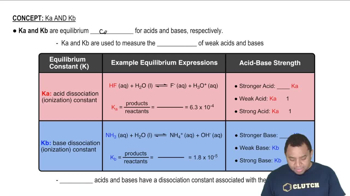
Caffeine (C8H10N4O2) is a weak base with a pKb of 10.4. Calculate the pH of a solution containing a caffeine concentration of 455 mg/L.
Write equations showing how each weak base ionizes water to form OH–. Also write the corresponding expression for Kb. a. NH3 b. HCO3– c. CH3NH2
Write equations showing how each weak base ionizes water to form OH–. Also write the corresponding expression for Kb. a. CO32–
Write equations showing how each weak base ionizes water to form OH–. Also write the corresponding expression for Kb. c. C2H5NH2
Determine the [OH–], pH, and pOH of a 0.15 M ammonia solution.