Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base according to the Arrhenius definition. a. HNO3(aq) b. NH4+(aq) d. HC2H3O2(aq)
Ch.16 - Acids and Bases

Chapter 16, Problem 34
Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base in aqueous solution according to the Arrhenius definition. a. NaOH(aq) b. H2SO4(aq) c. HBr(aq) d. Sr(OH)2(aq)
 Verified step by step guidance
Verified step by step guidance1
Identify the Arrhenius definition: An acid is a substance that increases the concentration of H+ ions in aqueous solution, while a base increases the concentration of OH- ions.
For NaOH(aq): Recognize that NaOH is a base because it dissociates in water to produce OH- ions. Write the equation: NaOH(aq) \rightarrow Na^+(aq) + OH^-(aq).
For H2SO4(aq): Recognize that H2SO4 is an acid because it dissociates in water to produce H+ ions. Write the equation: H2SO4(aq) \rightarrow 2H^+(aq) + SO4^{2-}(aq).
For HBr(aq): Recognize that HBr is an acid because it dissociates in water to produce H+ ions. Write the equation: HBr(aq) \rightarrow H^+(aq) + Br^-(aq).
For Sr(OH)2(aq): Recognize that Sr(OH)2 is a base because it dissociates in water to produce OH- ions. Write the equation: Sr(OH)2(aq) \rightarrow Sr^{2+}(aq) + 2OH^-(aq).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Arrhenius Definition of Acids and Bases
The Arrhenius definition states that acids are substances that increase the concentration of hydrogen ions (H⁺) in aqueous solution, while bases increase the concentration of hydroxide ions (OH⁻). This framework helps categorize substances based on their behavior in water, providing a straightforward way to identify acids and bases.
Recommended video:
Guided course
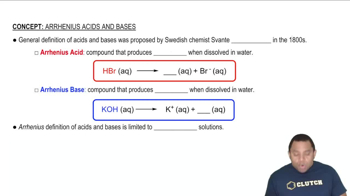
Arrhenius Acids and Bases
Dissociation in Aqueous Solution
Dissociation refers to the process by which an ionic or molecular compound separates into its constituent ions when dissolved in water. For example, NaOH dissociates into Na⁺ and OH⁻ ions, demonstrating its basic nature. Understanding dissociation is crucial for writing chemical equations that represent the behavior of acids and bases in solution.
Recommended video:
Guided course

Types of Aqueous Solutions
Chemical Equations for Acid-Base Reactions
Chemical equations represent the transformation of reactants into products during a chemical reaction. In the context of acids and bases, these equations illustrate how substances dissociate in water. For instance, H₂SO₄ dissociates to produce H⁺ and SO₄²⁻ ions, showcasing its acidic properties. Writing these equations is essential for demonstrating the Arrhenius definition in practice.
Recommended video:
Guided course
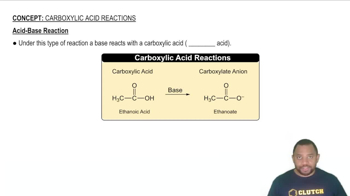
Acid-Base Reaction
Related Practice
Textbook Question
Textbook Question
Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base according to the Arrhenius definition. c. KOH(aq)
1
views
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. a. H2CO3(aq) + H2O(l) ⇌ H3O+(aq) + HCO3–(aq) c. HNO3(aq) + H2O(l) → H3O+(aq) + NO3–(aq)
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. b. NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
Textbook Question
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base. d. C5H5N(aq) + H2O(l) ⇌ C5H5NH+(aq) + OH–(aq)
