Textbook Question
Determine whether each cation is acidic or pH-neutral. For those cations that are acidic, write an equation that shows how the cation acts as an acid. c. Co3+

 Verified step by step guidance
Verified step by step guidance
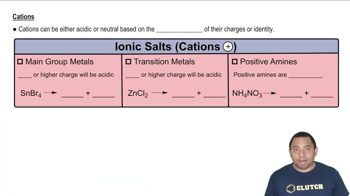
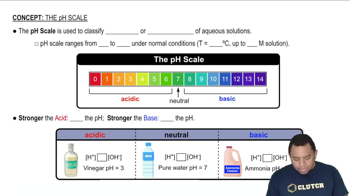
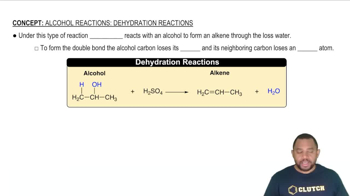
Determine whether each cation is acidic or pH-neutral. For those cations that are acidic, write an equation that shows how the cation acts as an acid. c. Co3+
Determine whether each cation is acidic or pH-neutral. For those cations that are acidic, write an equation that shows how the cation acts as an acid. d. CH2NH3+
Determine if each salt will form a solution that is acidic, basic, or pH-neutral. a. FeCl3 b. NaF c. CaBr2 d. NH4Br
Determine if each salt will form a solution that is acidic, basic, or pH-neutral. e. C6H5NH3NO2
Determine if each salt will form a solution that is acidic, basic, or pH-neutral. a. Al(NO3)3