Morphine has the formula C17H19NO3. It is a base and accepts one proton per molecule. It is isolated from opium. A 0.682-g sample of opium is found to require 8.92 mL of a 0.0116 M solution of sulfuric acid for neutralization. Assuming that morphine is the only acid or base present in opium, calculate the percent morphine in the sample of opium.
Ch.16 - Acids and Bases

Chapter 16, Problem 144
What is the concentration of ammonia in a solution prepared by dissolving 0.10 mol of acetic acid and 0.10 mol of ammonium chloride in enough water to make 1.0 L of solution?
 Verified step by step guidance
Verified step by step guidance1
Identify the relevant chemical reaction: Ammonium chloride (NH4Cl) dissociates in water to form ammonium ions (NH4+) and chloride ions (Cl-).
Recognize that ammonium ions (NH4+) can react with water to form ammonia (NH3) and hydronium ions (H3O+). This is an example of an acid-base equilibrium.
Write the equilibrium expression for the reaction: NH4+ + H2O ⇌ NH3 + H3O+. The equilibrium constant expression (Ka) for this reaction is Ka = [NH3][H3O+]/[NH4+].
Use the initial concentrations: Initially, [NH4+] = 0.10 M and [NH3] = 0 M. Assume x is the change in concentration at equilibrium, so [NH3] = x and [NH4+] = 0.10 - x.
Substitute the equilibrium concentrations into the Ka expression and solve for x, which represents the concentration of ammonia (NH3) at equilibrium.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Concentration
Concentration refers to the amount of a substance (solute) present in a given volume of solution. It is commonly expressed in moles per liter (Molarity, M), which is calculated by dividing the number of moles of solute by the volume of the solution in liters. Understanding concentration is essential for determining how much of a solute is available for reactions or interactions in a solution.
Recommended video:
Guided course
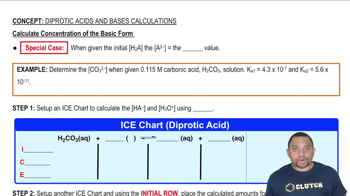
Calculate Concentration of the Basic Form
Acetic Acid and Ammonium Chloride in Solution
Acetic acid (CH₃COOH) is a weak acid that partially dissociates in solution, while ammonium chloride (NH₄Cl) is a salt that dissociates completely into ammonium ions (NH₄⁺) and chloride ions (Cl⁻). The presence of these two compounds in the solution affects the pH and the equilibrium of the system, which is important for understanding the behavior of ammonia (NH₃) in the solution.
Recommended video:
Guided course

Least Acidic Solution Example
Equilibrium and Ammonia Formation
In the context of this solution, ammonia can form from the dissociation of ammonium ions in equilibrium with acetic acid. The equilibrium constant (Ka) for acetic acid and the relationship between the concentrations of the species involved are crucial for calculating the concentration of ammonia. This concept is vital for understanding how the weak acid and its conjugate base interact in the solution.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
Write net ionic equations for the reactions that take place when aqueous solutions of the following substances are mixed: a. sodium cyanide and nitric acid b. ammonium chloride and sodium hydroxide c. sodium cyanide and ammonium bromide d. potassium hydrogen sulfate and lithium acetate e. sodium hypochlorite and ammonia
Textbook Question
The pH of a 1.00 M solution of urea, a weak organic base, is 7.050. Calculate the Ka of protonated urea.
Textbook Question
Lactic acid is a weak acid found in milk. Its calcium salt is a source of calcium for growing animals. A saturated solution of this salt, which we can represent as Ca(Lact)2, has a [Ca2+] = 0.26 M and a pH = 8.78. Assuming the salt is completely dissociated, calculate the Ka of lactic acid.
