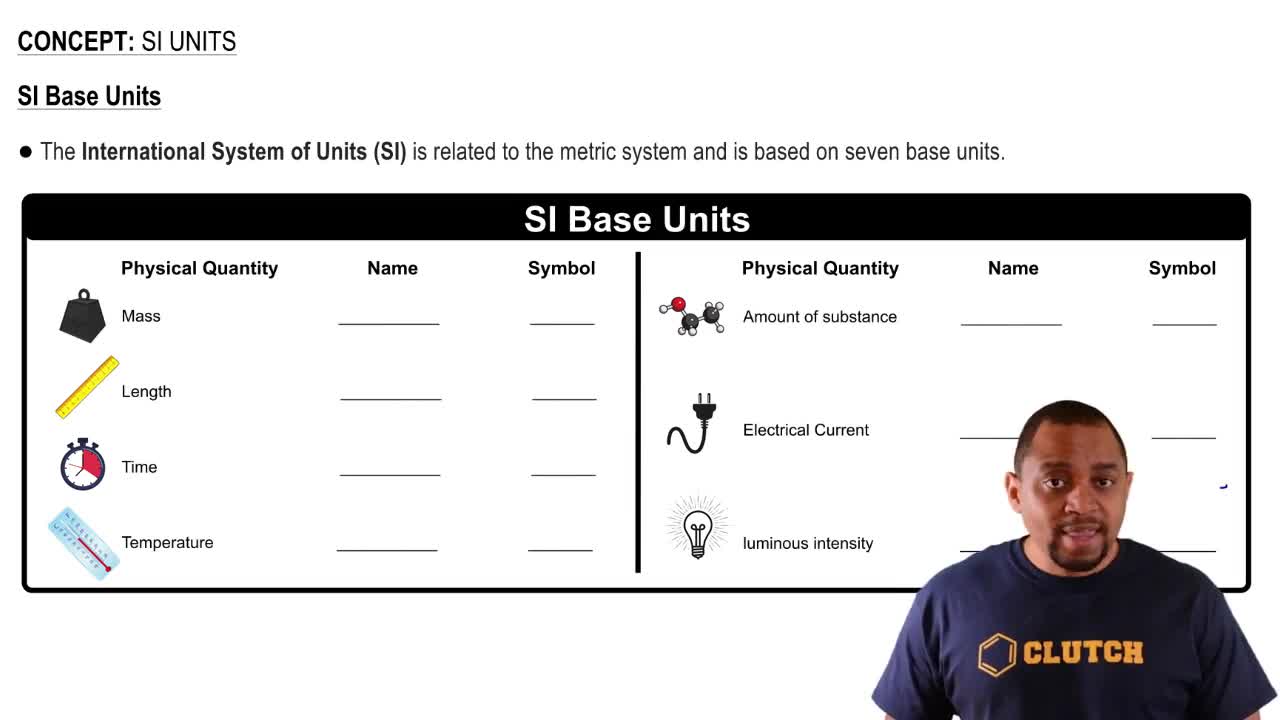
Textbook Question
Consider the reaction: N2(g) + 3 H2(g) ⇌ 2 NH3(g) Complete the table. Assume that all concentrations are equilibrium concentrations in M.
T (K) [N2] [H2] [NH3] Kc
500 0.115 0.105 0.439 _
575 0.110 _ 0.128 9.6
775 0.120 0.140 _ 0.0584