A certain substance X decomposes. Fifty percent of X remains after 100 minutes. How much X remains after 200 minutes if the reaction order with respect to X is (c) second order?
Ch.14 - Chemical Kinetics

Chapter 14, Problem 111
The energy of activation for the decomposition of 2 mol of HI to H2 and I2 in the gas phase is 185 kJ. The heat of formation of HI(g) from H2(g) and I2(g) is -5.65 kJ/mol. Find the energy of activation for the reaction of 1 mol of H2 and 1 mol of I2 to form 2 mol of HI in the gas phase.
 Verified step by step guidance
Verified step by step guidance1
Identify the given data: the activation energy for the decomposition of 2 mol of HI is 185 kJ, and the heat of formation of HI is -5.65 kJ/mol.
Understand that the activation energy for the reverse reaction (formation of HI) can be found using the relationship between the activation energies of the forward and reverse reactions and the enthalpy change of the reaction.
Calculate the enthalpy change (ΔH) for the formation of 2 mol of HI using the given heat of formation: ΔH = 2 mol × (-5.65 kJ/mol).
Use the equation for the activation energy of the reverse reaction: E_a(reverse) = E_a(forward) - ΔH, where E_a(forward) is the activation energy for the decomposition of HI.
Substitute the known values into the equation to find the activation energy for the formation of HI.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Activation Energy
Activation energy is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. In this context, the activation energy for the decomposition of HI is given, which is crucial for understanding the reverse reaction's energy requirements.
Recommended video:
Guided course

Activity Series Chart
Heat of Formation
The heat of formation is the change in enthalpy when one mole of a compound is formed from its elements in their standard states. It provides insight into the stability of compounds and is essential for calculating the energy changes in reactions. The heat of formation of HI is provided, which will be used to determine the energy change for the reverse reaction.
Recommended video:
Guided course

Enthalpy of Formation
Hess's Law
Hess's Law states that the total enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps, regardless of the pathway taken. This principle allows us to relate the activation energies of forward and reverse reactions, enabling the calculation of the activation energy for the formation of HI from H2 and I2 using the given data.
Recommended video:
Guided course
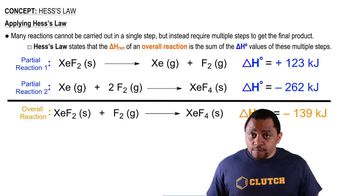
Hess's Law
Related Practice
Textbook Question
Textbook Question
The half-life for radioactive decay (a first-order process) of plutonium- 239 is 24,000 years. How many years does it take for one mole of this radioactive material to decay until just one atom remains?
Textbook Question
Ethyl chloride vapor decomposes by the first-order reaction: C2H5Cl → C2H4 + HCl The activation energy is 249 kJ/mol, and the frequency factor is 1.6⨉1014 s-1. Find the value of the rate constant at 710 K.
Textbook Question
Ethyl chloride vapor decomposes by the first-order reaction: C2H5Cl → C2H4 + HCl The activation energy is 249 kJ/mol, and the frequency factor is 1.6⨉1014 s-1. What fraction of the ethyl chloride decomposes in 15 minutes at this temperature?
