The half-life for the radioactive decay of C-14 is 5730 years and is independent of the initial concentration. How long does it take for 25% of the C-14 atoms in a sample of C-14 to decay?
Ch.14 - Chemical Kinetics

Chapter 14, Problem 61
The activation energy of a reaction is 56.8 kJ/mol and the frequency factor is 1.5⨉1011/ s. Calculate the rate constant of the reaction at 25 °C.
 Verified step by step guidance
Verified step by step guidance1
Convert the temperature from Celsius to Kelvin by adding 273.15 to the given temperature in Celsius: \( T = 25 + 273.15 \).
Use the Arrhenius equation to relate the rate constant \( k \) to the activation energy \( E_a \), frequency factor \( A \), and temperature \( T \): \( k = A \cdot e^{-E_a/(RT)} \).
Convert the activation energy from kJ/mol to J/mol by multiplying by 1000: \( E_a = 56.8 \times 1000 \).
Use the gas constant \( R = 8.314 \) J/(mol·K) in the Arrhenius equation.
Substitute the values for \( A \), \( E_a \), \( R \), and \( T \) into the Arrhenius equation and solve for \( k \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Activation Energy
Activation energy is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. In the context of the Arrhenius equation, a higher activation energy typically results in a slower reaction rate, as fewer molecules have sufficient energy to react at a given temperature.
Recommended video:
Guided course

Activity Series Chart
Arrhenius Equation
The Arrhenius equation relates the rate constant of a reaction to its activation energy and temperature. It is expressed as k = A * e^(-Ea/RT), where k is the rate constant, A is the frequency factor, Ea is the activation energy, R is the universal gas constant, and T is the temperature in Kelvin. This equation highlights how temperature and energy barriers influence reaction rates.
Recommended video:
Guided course
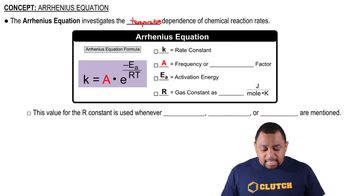
Arrhenius Equation
Rate Constant
The rate constant (k) is a proportionality factor in the rate law of a chemical reaction, indicating the speed at which the reaction occurs. It is influenced by factors such as temperature and activation energy. A higher rate constant signifies a faster reaction, while a lower rate constant indicates a slower reaction, making it a crucial parameter in chemical kinetics.
Recommended video:
Guided course
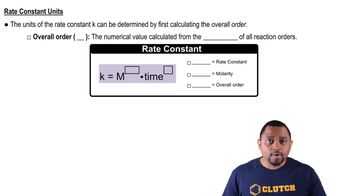
Rate Constant Units
Related Practice
Textbook Question
Textbook Question
The half-life for the radioactive decay of C-14 is 5730 years and is independent of the initial concentration. If a sample of C-14 initially contains 1.5 mmol of C-14, how many millimoles are left after 2255 years?
Textbook Question
The diagram shows the energy of a reaction as the reaction progresses. Label each blank box in the diagram.
a. reactants b. products c. activation energy (Ea) d. enthalpy of reaction (ΔHrxn)
Textbook Question
The rate constant (k) for a reaction was measured as a function of temperature. A plot of ln k versus 1/T (in K) is linear and has a slope of -7445 K. Calculate the activation energy for the reaction.
1
views
