This reaction is first order in N2O5: N2O5(g) → NO3(g) + NO2(g) The rate constant for the reaction at a certain temperature is 0.053/s. a. Calculate the rate of the reaction when [N2O5] = 0.055 M

A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero order in C. b. What is the overall order of the reaction?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
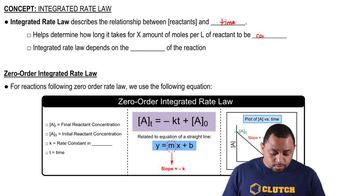
Reaction Order

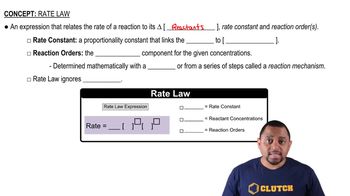
Rate Law
Zero Order Reactions
This reaction is first order in N2O5: N2O5(g) → NO3(g) + NO2(g) The rate constant for the reaction at a certain temperature is 0.053/s. b. What would the rate of the reaction be at the concentration indicated in part a if the reaction were second order? Zero order? (Assume the same numerical value for the rate constant with the appropriate units.)
A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero order in C.
a. Write a rate law for the reaction.
A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero order in C c. By what factor does the reaction rate change if [A] is doubled (and the other reactant concentrations are held constant)? d. By what factor does the reaction rate change if [B] is doubled (and the other reactant concentrations are held constant)? e. By what factor does the reaction rate change if [C] is doubled? f. By what factor does the reaction rate change if the concentrations of all three reactants are doubled?
A reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. a. Write a rate law for the reaction.
