Sodium hydroxide (NaOH) has a lattice energy of -887 kJ/mol and a heat of hydration of -932 kJ/mol. How much solution could be heated to boiling by the heat evolved by the dissolution of 25.0 g of NaOH? (For the solution, assume a heat capacity of 4.0 J/g·°C, an initial temperature of 25.0 °C, a boiling point of 100.0 °C, and a density of 1.05 g/mL.)

The Safe Drinking Water Act (SDWA) sets a limit for mercury—a toxin to the central nervous system—at 0.0020 ppm by mass. Water suppliers must periodically test their water to ensure that mercury levels do not exceed this limit. Suppose water becomes contaminated with mercury at twice the legal limit (0.0040 ppm). How much of this water would a person have to consume to ingest 50.0 mg of mercury?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Parts Per Million (ppm)
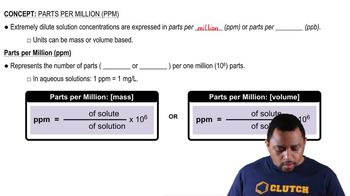
Mass and Volume Relationships

Toxicology and Safe Exposure Levels
A saturated solution forms when 0.0537 L of argon, at a pressure of 1.0 atm and temperature of 25 °C, is dissolved in 1.0 L of water. Calculate the Henry's law constant for argon.
A gas has a Henry's law constant of 0.112 M>atm. What total volume of solution is needed to completely dissolve 1.65 L of the gas at a pressure of 725 torr and a temperature of 25 °C?
Water softeners often replace calcium ions in hard water with sodium ions. Since sodium compounds are soluble, the presence of sodium ions in water does not cause the white, scaly residues caused by calcium ions. However, calcium is more beneficial to human health than sodium because calcium is a necessary part of the human diet, while high levels of sodium intake are linked to increases in blood pressure. The U.S. Food and Drug Administration (FDA) recommends that adults ingest less than 2.4 g of sodium per day. How many liters of softened water, containing a sodium concentration of 0.050% sodium by mass, would a person have to consume to exceed the FDA recommendation? (Assume a water density of 1.0 g/mL.)
