Household hydrogen peroxide is an aqueous solution containing 3.0% hydrogen peroxide by mass. What is the molarity of this solution? (Assume a density of 1.01 g/mL.)

An aqueous solution contains 5.0% NaCl by mass. How do you calculate the molality and mole fraction of the solution?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Molality
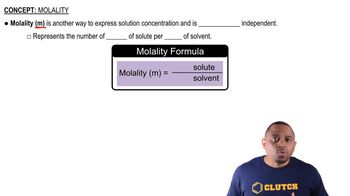
Mole Fraction

Mass Percent

One brand of laundry bleach is an aqueous solution containing 4.55% sodium hypochlorite (NaOCl) by mass. What is the molarity of this solution? (Assume a density of 1.02 g/mL.)
An aqueous solution contains 36% HCl by mass. Calculate the molality and mole fraction of the solution.
Which solution has the highest vapor pressure? a. 20.0 g of glucose (C6H12O6) in 100.0 mL of water b. 20.0 g of sucrose (C12H22O11) in 100.0 mL of water c. 10.0 g of potassium acetate KC2H3O2 in 100.0 mL of water
Calculate the vapor pressure of a solution containing 24.5 g of glycerin (C3H8O3) in 135 mL of water at 30.0 °C. The vapor pressure of pure water at this temperature is 31.8 torr. Assume that glycerin is not volatile and dissolves molecularly (i.e., it is not ionic), and use a density of 1.00 g/mL for the water.
