A solution contains 10.05 g of unknown compound dissolved in 50.0 mL of water. (Assume a density of 1.00 g/mL for water.) The freezing point of the solution is -3.16 °C. The mass percent composition of the compound is 60.97% C, 11.94% H, and the rest is O. What is the molecular formula of the compound?
Ch.13 - Solutions

Chapter 13, Problem 124
A 50.0-mL solution is initially 1.55% MgCl2 by mass and has a density of 1.05 g/mL. What is the freezing point of the solution after you add an additional 1.35 g MgCl2? (Use i = 2.5 for MgCl2.)
 Verified step by step guidance
Verified step by step guidance1
Calculate the initial mass of the solution using its volume and density: \( \text{mass} = \text{volume} \times \text{density} \).
Determine the initial mass of MgCl_2 in the solution using the percentage by mass: \( \text{mass of MgCl}_2 = \text{total mass} \times \frac{1.55}{100} \).
Add the additional 1.35 g of MgCl_2 to the initial mass of MgCl_2 to find the total mass of MgCl_2 in the solution.
Calculate the molality of the solution: \( \text{molality} = \frac{\text{moles of solute}}{\text{kilograms of solvent}} \). First, find moles of MgCl_2 using its molar mass, then find the mass of the solvent by subtracting the mass of MgCl_2 from the total mass of the solution.
Use the freezing point depression formula: \( \Delta T_f = i \cdot K_f \cdot m \), where \( i \) is the van't Hoff factor, \( K_f \) is the freezing point depression constant for water, and \( m \) is the molality, to find the change in freezing point. Subtract \( \Delta T_f \) from the normal freezing point of water to find the new freezing point.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
6mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Colligative Properties
Colligative properties are physical properties of solutions that depend on the number of solute particles in a given amount of solvent, rather than the identity of the solute. These properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. In this question, the freezing point depression is relevant as it quantifies how the addition of solute affects the freezing point of the solution.
Recommended video:
Guided course

Colligative Properties
Freezing Point Depression
Freezing point depression is a colligative property that describes the decrease in the freezing point of a solvent when a solute is added. The extent of freezing point depression can be calculated using the formula ΔTf = i * Kf * m, where ΔTf is the change in freezing point, i is the van 't Hoff factor (number of particles the solute dissociates into), Kf is the freezing point depression constant of the solvent, and m is the molality of the solution.
Recommended video:
Guided course

Freezing Point Depression
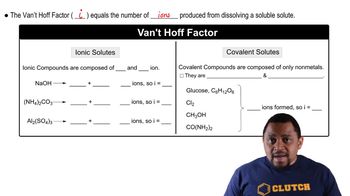
Van 't Hoff Factor (i)
The van 't Hoff factor (i) is a dimensionless number that indicates the number of particles into which a solute dissociates in solution. For ionic compounds like MgCl2, which dissociates into one Mg²⁺ ion and two Cl⁻ ions, the van 't Hoff factor is 2.5 in this case, accounting for the degree of ionization and any association that may occur in solution, which is crucial for accurately calculating colligative properties.
Recommended video:
Guided course
Van't Hoff Factor
Related Practice
Textbook Question
Textbook Question
The osmotic pressure of a solution containing 2.10 g of an unknown compound dissolved in 175.0 mL of solution at 25 °C is 1.93 atm. The combustion of 24.02 g of the unknown compound produced 28.16 g CO2 and 8.64 g H2O. What is the molecular formula of the compound (which contains only carbon, hydrogen, and oxygen)?
Textbook Question
A 100.0-mL aqueous sodium chloride solution is 13.5% NaCl by mass and has a density of 1.12 g/mL. What would you add (solute or solvent) and what mass of it to make the boiling point of the solution 104.4 °C? (Use i = 1.8 for NaCl.)
