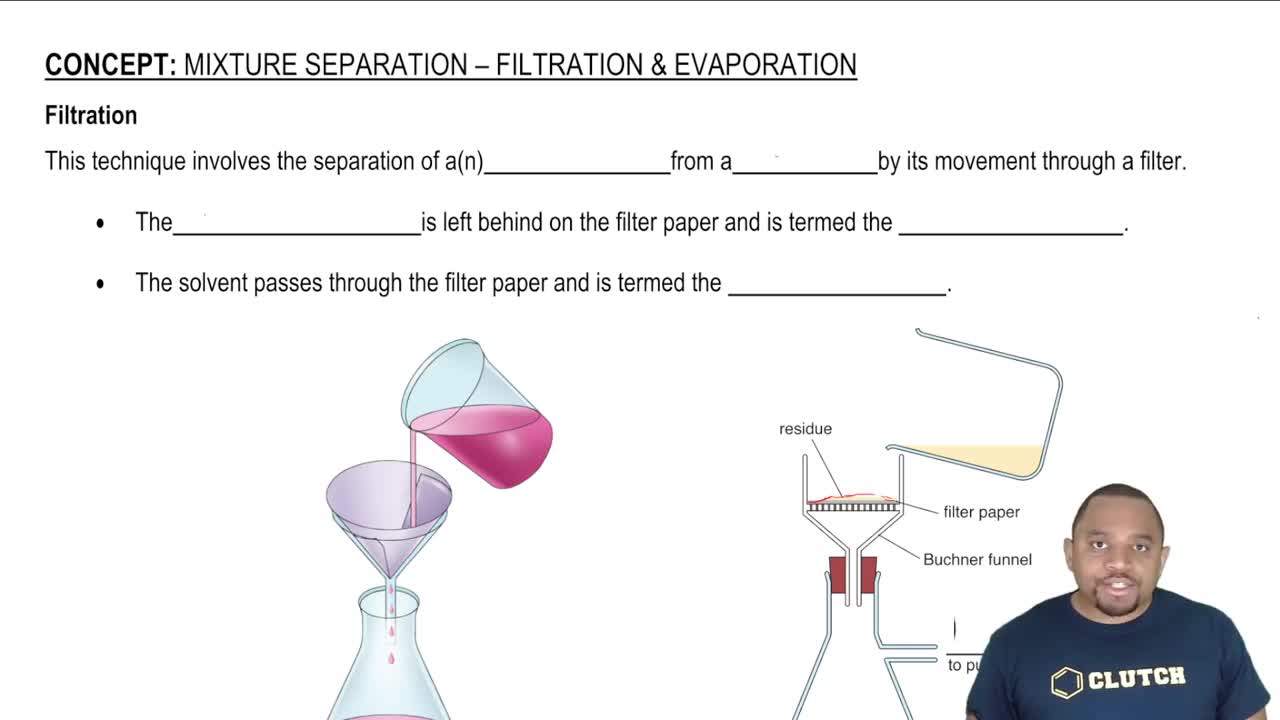
Textbook Question
When a thin glass tube is put into water, the water rises 1.4 cm. When the same tube is put into hexane, the hexane rises only 0.4 cm. Explain.

 Verified step by step guidance
Verified step by step guidance

When a thin glass tube is put into water, the water rises 1.4 cm. When the same tube is put into hexane, the hexane rises only 0.4 cm. Explain.
Which evaporates more quickly: 55 mL of water (H2O) in a beaker or 100 mL of acetone [(CH3)2CO] in an identical beaker under identical conditions? Is the vapor pressure of the two substances different? Explain.