Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. c. C2H6 (skeletal structure H3CCH3)
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory

All textbooks Tro 4th Edition
Tro 4th Edition Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory Problem 69
Problem 69
 Tro 4th Edition
Tro 4th Edition Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory Problem 69
Problem 69Chapter 10, Problem 69
Sketch the bonding molecular orbital that results from the linear combination of two 1s orbitals. Indicate the region where interference occurs and state the kind of interference (constructive or destructive).
 Verified step by step guidance
Verified step by step guidance1
Identify the two 1s atomic orbitals that will combine. Each 1s orbital is spherically symmetrical around the nucleus of an atom.
Understand that when these orbitals overlap, they can do so in two ways: constructively (in-phase combination) or destructively (out-of-phase combination).
For constructive interference, the wave functions of the two 1s orbitals add together, leading to an increase in electron density between the nuclei. This is the bonding molecular orbital.
Sketch the bonding molecular orbital: Draw two overlapping circles (representing the 1s orbitals) with the overlap area shaded more heavily to indicate increased electron density.
Indicate the region of constructive interference, which is the area where the two orbitals overlap. Label this region as the area of constructive interference, which is responsible for the bonding character of the molecular orbital.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Orbital Theory
Molecular Orbital Theory describes how atomic orbitals combine to form molecular orbitals, which can be occupied by electrons in a molecule. When two atomic orbitals, such as 1s orbitals from two hydrogen atoms, combine, they can form two types of molecular orbitals: bonding and antibonding. The bonding molecular orbital is lower in energy and stabilizes the molecule, while the antibonding orbital is higher in energy and can destabilize it.
Recommended video:
Guided course
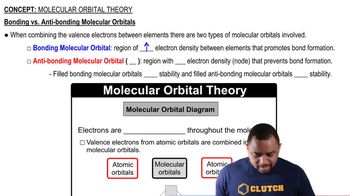
Molecular Orbital Theory
Linear Combination of Atomic Orbitals (LCAO)
The Linear Combination of Atomic Orbitals (LCAO) is a method used to construct molecular orbitals by combining the wave functions of atomic orbitals. In the case of two 1s orbitals, the LCAO results in a bonding molecular orbital formed by the addition of the two wave functions, leading to a region of increased electron density between the nuclei. This combination can also produce an antibonding orbital through subtraction of the wave functions.
Recommended video:
Guided course
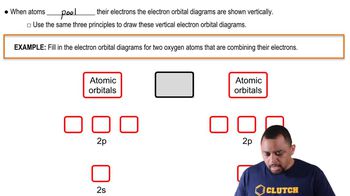
Atomic Orbitals Example
Interference of Waves
Interference of waves occurs when two or more wave functions overlap, leading to regions of constructive or destructive interference. Constructive interference happens when wave peaks align, resulting in increased amplitude, while destructive interference occurs when a peak aligns with a trough, leading to reduced amplitude. In the context of molecular orbitals, constructive interference in the bonding orbital enhances electron density between the nuclei, while destructive interference in the antibonding orbital reduces it.
Recommended video:
Guided course
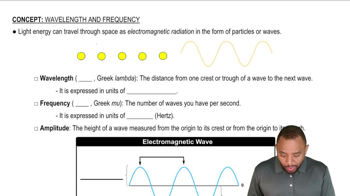
Electromagnetic Waves
Related Practice
Textbook Question
Textbook Question
Consider the structure of the amino acid alanine. Indicate the hybridization about each interior atom.
Textbook Question
Consider the structure of the amino acid aspartic acid. Indicate the hybridization about each interior atom.
Textbook Question
Draw an MO energy diagram and predict the bond order of Be2+ and Be2- . Do you expect these molecules to exist in the gas phase?
1
views
Textbook Question
Draw an MO energy diagram and predict the bond order of Li2+ and Li2-. Do you expect these molecules to exist in the gas phase?
1
views
Textbook Question
Sketch the bonding and antibonding molecular orbitals that result from linear combinations of the 2px atomic orbitals in a homonuclear diatomic molecule. (The 2px orbitals are those whose lobes are oriented along the bonding axis.)