Most vitamins can be classified as either fat soluble, which results in their tendency to accumulate in the body (so that taking too much can be harmful), or water soluble, which results in their tendency to be quickly eliminated from the body in urine. Examine the structural formulas and space-filling models of these vitamins and determine whether each one is fat soluble (mostly nonpolar) or water soluble (mostly polar). (a) vitamin C

 Tro 4th Edition
Tro 4th Edition Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory
Ch.10 - Chemical Bonding II: Molecular Shapes & Valence Bond Theory Problem 91
Problem 91Draw a molecular orbital energy diagram for ClF. (Assume that the sp orbitals are lower in energy than the p orbitals.) What is the bond order in ClF?
 Verified step by step guidance
Verified step by step guidance
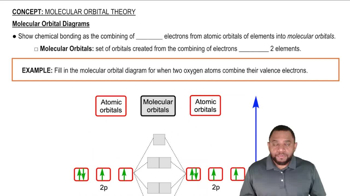
Verified video answer for a similar problem:
Key Concepts
Molecular Orbital Theory

Bond Order

Energy Levels of Orbitals
Most vitamins can be classified as either fat soluble, which results in their tendency to accumulate in the body (so that taking too much can be harmful), or water soluble, which results in their tendency to be quickly eliminated from the body in urine. Examine the structural formulas and space-filling models of these vitamins and determine whether each one is fat soluble (mostly nonpolar) or water soluble (mostly polar). (c) niacin (vitamin B3)
Water does not easily remove grease from dishes or hands because grease is nonpolar and water is polar. The addition of soap to water, however, allows the grease to dissolve. Study the structure of sodium stearate (a soap) and describe how it works.
Draw Lewis structures and MO diagrams for CN+ , CN, and CN- . According to the Lewis model, which species is most stable?
The compound C3H4 has two double bonds. Describe its bonding and geometry, using a valence bond approach.