Textbook Question
Complete the table. a. 1245 kg _ 1.245×106 g _ 1.245×109 mg
2
views

 Verified step by step guidance
Verified step by step guidance
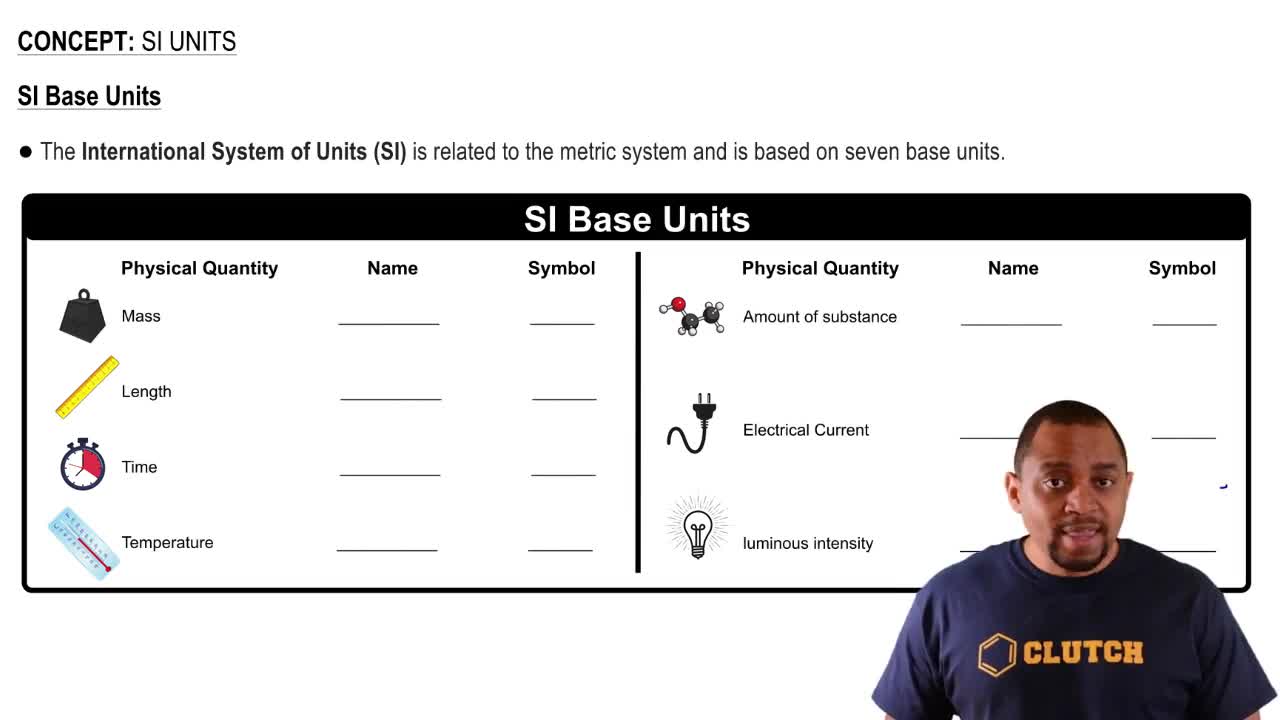


Complete the table. a. 1245 kg _ 1.245×106 g _ 1.245×109 mg
Complete the table. b. 515 km _____dm _____cm
Complete the table. c. 122.355 s _____ms _____ks
Complete the table. a. 355 km/s _____cm/s _____m/ms
Complete the table. b. 1228 g/l _____g/ml _____kg/ml d. 2.554 mg/ml _____g/l _____mg/ml
Complete the table. c. 554 mK/s _____K/s _____mK/ms