Textbook Question
Which numbers are exact (and therefore have an unlimited number of significant figures)? b. 12 in = 1 ft
1
views

 Verified step by step guidance
Verified step by step guidance

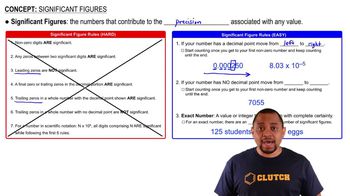

Which numbers are exact (and therefore have an unlimited number of significant figures)? b. 12 in = 1 ft
Round each number to three significant figures. a. 79,845.82 b. 1.548937×107 c. 2.3499999995 d. 0.000045389
Calculate to the correct number of significant figures. a. 89.3 × 77.0 × 0.08
Calculate to the correct number of significant figures. b. (5.01×105) / (7.8×102)
Calculate to the correct number of significant figures. c. 4.005 × 74 × 0.007