The Toyota Prius, a hybrid electric vehicle, has an EPA gas mileage rating of 52 mi/gal in the city. How many kilometers can the Prius travel on 15 L of gasoline?

A sample of gaseous neon atoms at atmospheric pressure and 0 °C contains 2.69×1022 atoms per liter. The atomic radius of neon is 69 pm. What fraction of the space do the atoms themselves occupy? What does this reveal about the separation between atoms in the gaseous phase?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
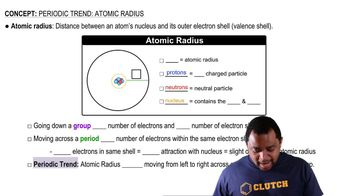
Atomic Radius
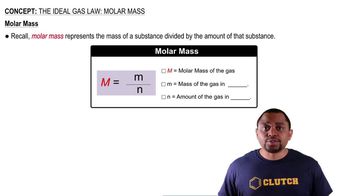
Molar Volume of a Gas
Fraction of Space Occupied by Atoms

The Honda Insight, a hybrid electric vehicle, has an EPA gas mileage rating of 41 mi/gal in the city. How many kilometers can the Insight travel on the amount of gasoline that would fit in a soda can? The volume of a soda can is 355 mL.
The single proton that forms the nucleus of the hydrogen atom has a radius of approximately 1.0×10−13 cm. The hydrogen atom itself has a radius of approximately 52.9 pm. What fraction of the space within the atom is occupied by the nucleus?
The diameter of a hydrogen atom is 212 pm. Find the length in kilometers of a row of 6.02×1023 hydrogen atoms. The diameter of a ping pong ball is 4.0 cm. Find the length in kilometers of a row of 6.02×1023 ping pong balls.
The world's record in the 100-m dash is 9.69 s, and in the 100-yd dash it is 9.21 s. Find the speed in mi/hr of the runners who set these records. (Assume three significant figures for 100 m and 100 yd.)
The daily recommended intake of calcium for an average adult is 1,000 mg. There is 125 mg of calcium in 100 grams of milk. If a 150 g smoothie contains 75 grams of milk, how many grams of smoothie should an adult consume to meet the daily recommended intake?
