Textbook Question
Which solid has the highest melting point? Why? C(s, diamond), Kr(s), NaCl(s), H2O(s)

 Verified step by step guidance
Verified step by step guidance


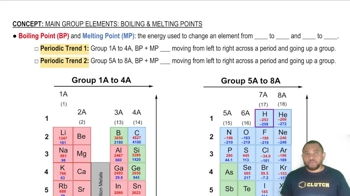
Which solid has the highest melting point? Why? C(s, diamond), Kr(s), NaCl(s), H2O(s)
Which solid in each pair has the higher melting point and why?
a. TiO2(s) or HOOH(s)
b. CCl4(s) or SiCl4(s)
c. Kr(s) or Xe(s)
Which solid in each pair has the higher melting point and why? d. NaCl(s) or CaO(s)
An oxide of rhenium crystallizes with the unit cell shown here (rhenium=gray; oxygen=red). What is the formula of the oxide?
Identify the structure of each of the two unit cells shown in Problem 48 as the rock salt structure, zinc blende structure, fluorite structure, antifluorite structure, or none of these.