Molybdenum crystallizes with the body-centered unit cell. The radius of a molybdenum atom is 136 pm. Calculate the edge length of the unit cell and the density of molybdenum
Ch.12 - Solids and Modern Material

Chapter 12, Problem 36
Barium has a density of 3.59 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a barium atom.
 Verified step by step guidance
Verified step by step guidance1
Identify the type of unit cell: Barium crystallizes in a body-centered cubic (BCC) unit cell.
Recall the relationship between the edge length (a) of a BCC unit cell and the atomic radius (r): In a BCC structure, the body diagonal is equal to 4 times the atomic radius, i.e., \( \sqrt{3}a = 4r \).
Calculate the mass of one unit cell: Use the density formula \( \text{Density} = \frac{\text{Mass}}{\text{Volume}} \) to find the mass of the unit cell. The volume of the unit cell is \( a^3 \), and the density is given as 3.59 g/cm³.
Determine the number of atoms per unit cell: In a BCC unit cell, there are 2 atoms per unit cell.
Use the mass of one barium atom and the number of atoms per unit cell to find the edge length (a), then solve for the atomic radius (r) using the relationship \( \sqrt{3}a = 4r \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Density and its Relation to Volume
Density is defined as mass per unit volume (density = mass/volume). In this context, knowing the density of barium allows us to calculate the volume occupied by a certain mass of barium atoms. This volume is crucial for determining the size of the unit cell and, subsequently, the radius of the barium atom.
Recommended video:
Guided course

Density Concepts
Unit Cell and Atomic Radius in Crystallography
A unit cell is the smallest repeating unit in a crystal lattice that reflects the overall symmetry and structure of the crystal. In a body-centered cubic (BCC) structure, there are two atoms per unit cell, and the relationship between the atomic radius and the unit cell dimensions can be used to derive the radius of the barium atom based on the geometry of the BCC arrangement.
Recommended video:
Guided course
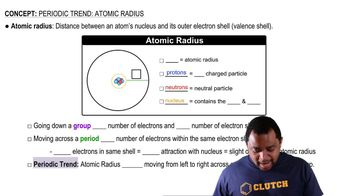
Atomic Radius
Mathematical Relationships in Crystallography
In a body-centered cubic lattice, the relationship between the atomic radius (r) and the edge length (a) of the unit cell is given by the equation a = 4r/√3. This relationship allows us to calculate the atomic radius once we determine the edge length from the volume derived from the density and mass of barium, linking the macroscopic properties of the material to its atomic structure.
Recommended video:
Guided course
Kp vs Kc Relationship
Related Practice
Textbook Question
Textbook Question
Platinum crystallizes with the face-centered cubic unit cell. The radius of a platinum atom is 139 pm. Calculate the edge length of the unit cell and the density of platinum in g/cm3.
1
views
Textbook Question
Rhodium has a density of 12.41 g/cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.
Textbook Question
Plonium crystallizes with a simple cubic structure. It has a density of 9.3 g/cm3, a radius of 167 pm, and a molar mass of 209 g/mol. Use these data to calculate Avogadro's number (the number of atoms in one mole).
Textbook Question
Identify each solid as molecular, ionic, or atomic. a. Ar(s) b. H2O(s) c. K2O(s) d. Fe(s)
