Textbook Question
Write balanced equations for the formation of the following compounds from their elements. (a) Iron(III) oxide

 Verified step by step guidance
Verified step by step guidance


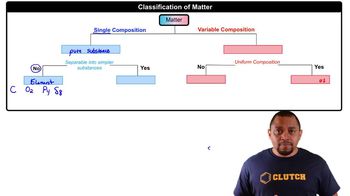
Write balanced equations for the formation of the following compounds from their elements. (a) Iron(III) oxide
Write balanced equations for the formation of the following compounds from their elements. (b) Sucrose (table sugar, C12H22O11)
Write balanced equations for the formation of the following compounds from their elements. (a) Ethanol (C2H6O)
Write balanced equations for the formation of the following compounds from their elements. (c) Dichloromethane (a liquid, CH2Cl2)