Textbook Question
Redraw the following diagram to represent the situation (b) when work has been lost by the system.

 Verified step by step guidance
Verified step by step guidance


Redraw the following diagram to represent the situation (b) when work has been lost by the system.
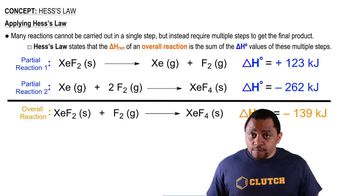
The reaction of A with B to give D proceeds in two steps: (1) A + B → C ΔH° = -20 kJ (2) C + B → D ΔH° = +50 kJ (3) A + 2B → D ΔH° = ? (a) Which Hess's law diagram represents the reaction steps and the overall reaction? Diagram 1 Diagram 2
The following reaction is exothermic:
(a) Write a balanced equation for the reaction (red spheres represent A atoms and ivory spheres represent B atoms)
The following reaction is exothermic: (c) Is the reaction likely to be spontaneous at lower temperatures only, at higher temperatures only, or at all temperatures?