Textbook Question
Which has more kinetic energy, a 1400-kg car moving at115 km/h or a 12,000-kg truck moving at 38 km/h?
1
views

 Verified step by step guidance
Verified step by step guidance

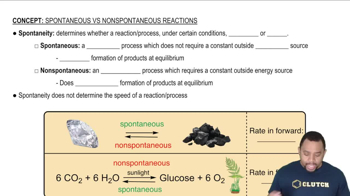
The following reaction is exothermic:
(a) Write a balanced equation for the reaction (red spheres represent A atoms and ivory spheres represent B atoms)
The following reaction is exothermic: (c) Is the reaction likely to be spontaneous at lower temperatures only, at higher temperatures only, or at all temperatures?