Textbook Question
Use the data in Appendix B to find standard enthalpies of reaction in kilojoules for the following processes: (c) Fe2O3 (s) + 3 CO (g) → Fe(s) + 3 CO2(g)

 Verified step by step guidance
Verified step by step guidance
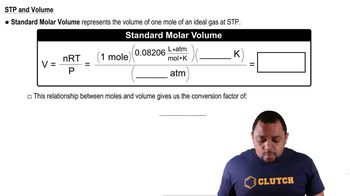
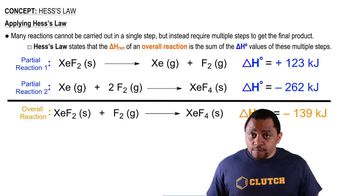

Use the data in Appendix B to find standard enthalpies of reaction in kilojoules for the following processes: (c) Fe2O3 (s) + 3 CO (g) → Fe(s) + 3 CO2(g)
Use the data in Appendix B to find standard enthalpies of reaction in kilojoules for the following processes: (a) C(s) + CO2(g) → 2 CO(g)
Use the data in Appendix B to find standard enthalpies of reaction in kilojoules for the following processes: (b) 2 H2O2 (aq) → 2 H2O (l) + O2(g)
Isooctane, C8H18, is the component of gasoline from which the term octane rating derives. (a) Write a balanced equation for the combustion of isooctane(l) with O2 to yield CO2(g) and H2O(l)
What does entropy measure?