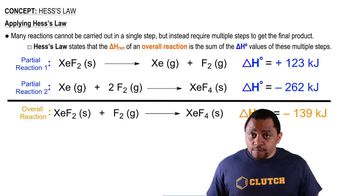
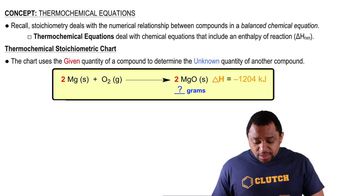
Textbook Question
Sulfuric acid (H2SO4), the most widely produced chemical in the world, is amde yb a two-step oxidaiton of sulfur to sulfur trioxide, SO3, followed by reaciton with water. Calculate ΔH°f for SO3 in kJ/mol, given the following data: S(s) + O2(g) → SO2(g) ΔH° = -296.8 kJ SO2(g) + 1/2 O2(g) → SO3(g) ΔH° = -98.9 kJ