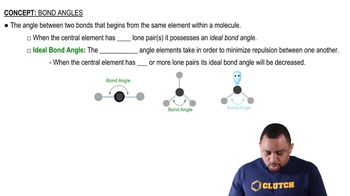
Textbook Question
What is the difference in spatial distribution between electrons in a p bond and electrons in a s bond?
1
views

 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 64
Problem 64
 Verified step by step guidance
Verified step by step guidance

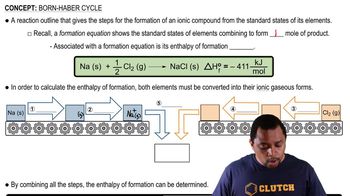
Bupropion, marketed as Wellbutrin, is a heavily prescribed medication used in the treatment of depression. Complete the following electron-dot structure for Bupropion by adding lone pairs of electrons.
(a) How many s and how many p bonds are in the molecule?