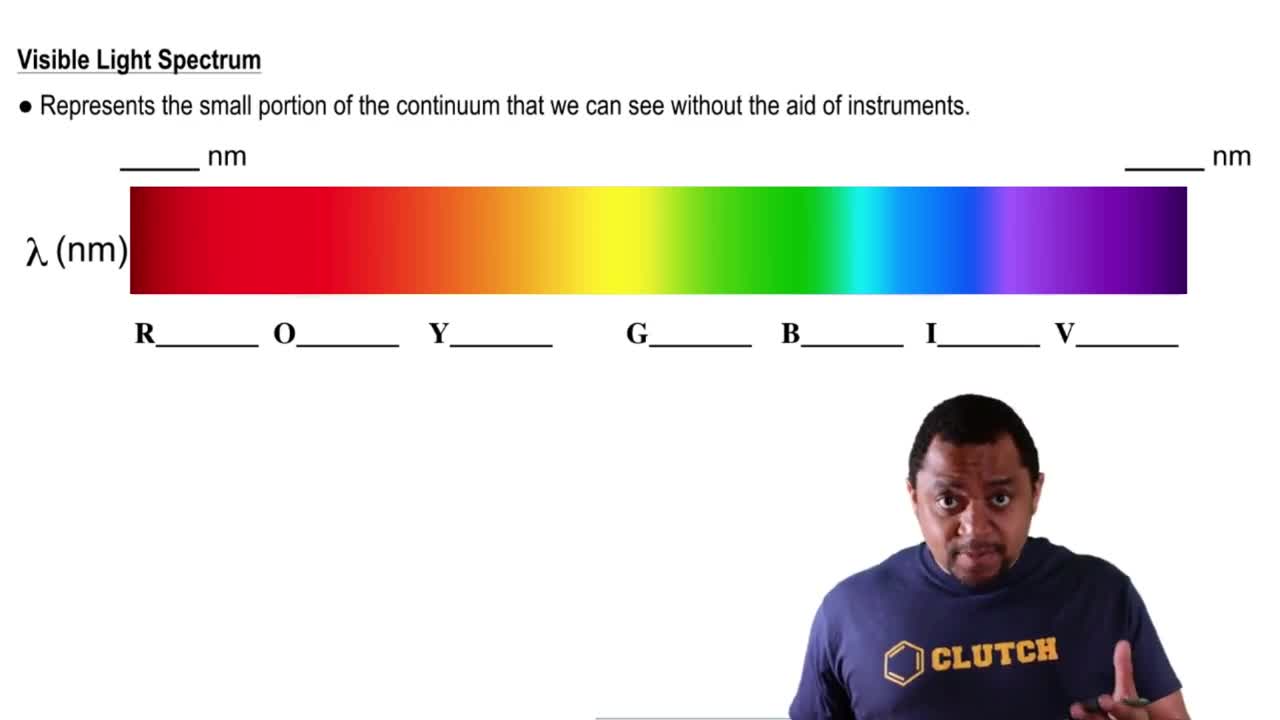
Cyclooctatetraene dianion, C8H82-, is an organic ion with the structure shown. Considering only the p bonds and not the s bonds, cyclooctatetraene dianion can be described by the following energy diagrams of its p molecular orbitals:
(b) Three of the p molecular orbitals are bonding, three are antibonding, and two are nonbonding, meaning that they have the same energy level as isolated p orbitals. Which is which?