Textbook Question
Take a guess. What do you think is a likely ground-state electron configuration for the sodium ion, Na+, formed by loss of an electron from a neutral sodium atom?

 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 104b
Problem 104b Verified step by step guidance
Verified step by step guidance
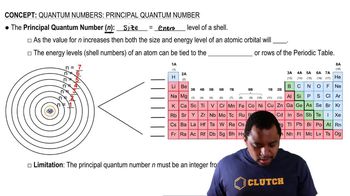
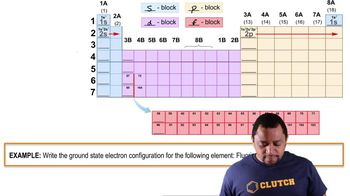
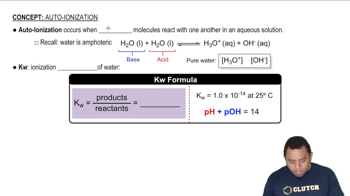
Consider the electronic structure of the element bismuth. (a) The first ionization energy of bismuth is Ei1 = +703 kJ/ mol. What is the longest possible wavelength of light that could ionize an atom of bismuth?
Iron is commonly found as Fe, Fe2++, and Fe3+. (c) The third ionization energy of Fe is Ei3 = +2952 kJ/mol. What is the longest wavelength of light that could ionize Fe2+(g) to Fe3+(g)?