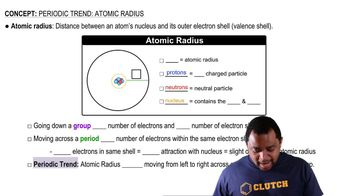
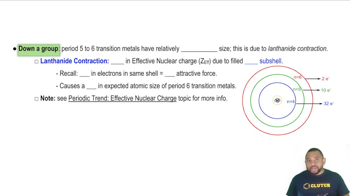
Textbook Question
Imagine a universe in which the four quantum numbers can have the same possible values as in our universe except that the angular-momentum quantum number l can have integral values of 0, 1, 2...n + 1 (instead of 0, 1, 2..., n - 1). (a) How many elements would be in the first two rows of the periodic table in this universe?