Write the symbol, give the ground-state electron configuration, and draw an orbital-filling diagram for each of the following atoms. Use the abbreviation of the preceding noble gas to represent the inner-shell electrons. (a) The heaviest alkaline earth metal
Ch.5 - Periodicity & Electronic Structure of Atoms

Chapter 5, Problem 111
Given the subshells 1s, 2s, 2p, 3s, 3p, and 3d, identify those that meet the following descriptions: (c) Is empty in a nitrogen atom (d) Is full in a carbon atom.
 Verified step by step guidance
Verified step by step guidance1
Identify the electron configuration of a nitrogen atom. Nitrogen has an atomic number of 7, so its electron configuration is 1s² 2s² 2p³.
Determine which subshells are empty in a nitrogen atom. From the electron configuration, the 1s and 2s subshells are full, and the 2p subshell has 3 electrons. The 3s, 3p, and 3d subshells are not occupied in a nitrogen atom, so they are empty.
Identify the electron configuration of a carbon atom. Carbon has an atomic number of 6, so its electron configuration is 1s² 2s² 2p².
Determine which subshells are full in a carbon atom. From the electron configuration, the 1s and 2s subshells are full, while the 2p subshell is partially filled with 2 electrons.
Conclude that for a nitrogen atom, the 3s, 3p, and 3d subshells are empty, and for a carbon atom, the 1s and 2s subshells are full.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's orbitals. For nitrogen (atomic number 7), the electron configuration is 1s² 2s² 2p³, indicating that the 3s, 3p, and 3d subshells are empty. In contrast, carbon (atomic number 6) has the configuration 1s² 2s² 2p², meaning its 3s, 3p, and 3d subshells are also empty.
Recommended video:
Guided course
Electron Configuration Example
Subshell Filling Order
Electrons fill subshells according to the Aufbau principle, which states that they occupy the lowest energy orbitals first. The order of filling is determined by the increasing energy levels of the subshells, typically following the sequence 1s, 2s, 2p, 3s, 3p, and then 3d. This principle helps in predicting which subshells are filled or empty in various elements.
Recommended video:
Guided course

Bond Order Example
Valence Electrons
Valence electrons are the outermost electrons of an atom and are crucial for determining chemical properties and bonding behavior. In nitrogen, the valence electrons are in the 2s and 2p subshells, while carbon has its valence electrons in the same subshells. Understanding the distribution of valence electrons helps in identifying which subshells are full or empty in different elements.
Recommended video:
Guided course
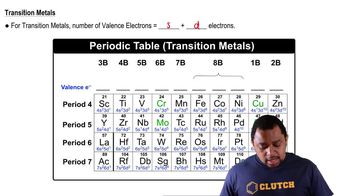
Transition Metals Valence Electrons
Related Practice
Textbook Question
Textbook Question
Write the symbol, give the ground-state electron configuration, and draw an orbital-filling diagram for each of the following atoms. Use the abbreviation of the preceding noble gas to represent the inner-shell electrons.
(c) The heaviest actinide metal
Textbook Question
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (a) Has l = 2
Textbook Question
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (b) Can have ml = -1
Textbook Question
Given the subshells 1s, 2s, 2p, 3s, 3p and 3d, identify those that meet the following descriptions. (e) Contains the outermost electrons in a beryllium atom
