Textbook Question
Which two of the four quantum numbers determine the energy level of an orbital in a multielectron atom?
1
views
 Verified step by step guidance
Verified step by step guidance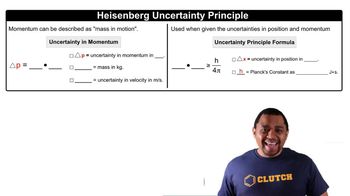

Give the expected ground-state electron configurations for atoms with the following atomic numbers. (a) Z = 55 (b) Z = 40 (c) Z = 80 (d) Z = 62