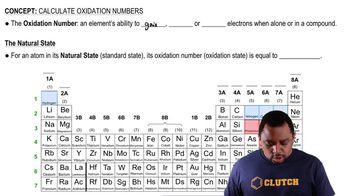
Element M is prepared industrially by a two-step procedure according to the following (unbalanced) equations:
Assume that 0.855 g of M2O3 is submitted to the reaction sequence. When the HCl produced in step (2) is dissolved in water and titrated with 0.511 M NaOH, 144.2 mL of the NaOH solution is required to neutralize the HCl. (a) Balance both equations.