Textbook Question
How many grams of solute would you use to prepare each of the following solutions? (b) 167 mL of 0.200 M boric acid (H3BO3)
2
views

 Verified step by step guidance
Verified step by step guidance


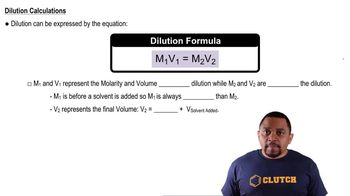
How many grams of solute would you use to prepare each of the following solutions? (b) 167 mL of 0.200 M boric acid (H3BO3)
How many milliliters of a 0.45 M BaCl2 solution contain 15.0 g of BaCl2?
Write balanced net ionic equations for the following reactions in acidic solution. (a) Zn(s) + VO2+(aq) → Zn2+(aq) + V3+(aq)