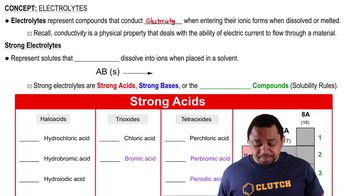
Textbook Question
Individual solutions of Ba(OH)2 and H2SO4 both conduct electricity, but the conductivity disappears when equal molar amounts of the solutions are mixed. Explain.

 Verified step by step guidance
Verified step by step guidance


What is the total molar concentration of ions in each of the following solutions, assuming complete dissociation? (a) A 0.750 M solution of K2CO3
What is the total molar concentration of ions in each of the following solutions, assuming complete dissociation? (b) A 0.355 M solution of AlCl3
What is the total molar concentration of ions in each of the following solutions? (a) A 1.250 M solution of CH3OH