Textbook Question
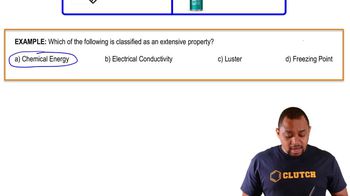
The concentration of an aqueous solution of NaOCl (sodium hypochlorite; the active ingredient in household bleach) can be determined by a redox titration with iodide ion in acidic solution: Assume that the blue spheres in the buret represent I-ions, the red spheres in the flask represent OCl-ions, the con-centration of the I-ions in the buret is 0.120 M, and the volumes in the buret and the flask are identical. What is the concentration of NaOCl in the flask? What percentage of the I-solution in the buret must be added to the flask to react with all the OCl-ions?