Textbook Question
Cyclohexane, C6H12, is an important starting material used in the industrial synthesis of nylon. Each carbon has four covalent bonds, two to hydrogen and two to other carbons. Draw the structural formula of cyclohexane.

 Verified step by step guidance
Verified step by step guidance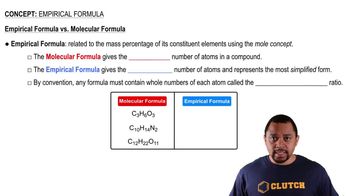

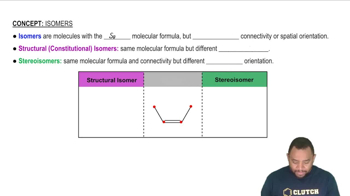
Isooctane, the substance in gasoline from which the term octane rating derives, has the formula C8H18. Each carbon has four covalent bonds, and the atoms are connected in the sequence shown. Draw the complete structural formula of isooctane.