Ch.23 - Organic and Biological Chemistry

Chapter 23, Problem 76
(a) Draw an orbital overlap picture of ethane (CH3CH3), assuming both carbon atoms are sp3 hybridized. What are the C–H bond angles in this drawing? (b) Draw an orbital overlap picture of ethane, assuming both carbon atoms are sp2 hybridized. What are the C–H bond angles in this drawing? (c) The real structure of ethane is like the picture you drew in part (a). Use VSEPR theory to explain why your picture from part (a) makes a more stable molecule than your picture in part (b).
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the hybridization concept. In ethane (CH3CH3), each carbon atom forms four sigma bonds, which suggests sp3 hybridization. This involves mixing one s orbital and three p orbitals to form four equivalent sp3 hybrid orbitals.
Step 2: Draw the orbital overlap for sp3 hybridized ethane. Each carbon atom uses its sp3 hybrid orbitals to form sigma bonds with three hydrogen atoms and one sigma bond with the other carbon atom. The C-H bond angles in this configuration are approximately 109.5 degrees, which is characteristic of a tetrahedral geometry.
Step 3: Consider the sp2 hybridization scenario. If both carbon atoms in ethane were sp2 hybridized, each carbon would use three sp2 hybrid orbitals to form sigma bonds and one unhybridized p orbital for a pi bond. This would result in a planar structure with C-H bond angles of approximately 120 degrees.
Step 4: Apply VSEPR theory to explain stability. VSEPR (Valence Shell Electron Pair Repulsion) theory states that electron pairs around a central atom will arrange themselves to minimize repulsion. In the sp3 hybridized ethane, the tetrahedral arrangement minimizes electron pair repulsion, making it more stable than the planar sp2 configuration.
Step 5: Conclude with the real structure. The real structure of ethane is sp3 hybridized, as shown in part (a), because it provides a more stable, lower energy configuration due to minimized electron pair repulsion, as explained by VSEPR theory.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Hybridization
Hybridization is the process of mixing atomic orbitals to form new hybrid orbitals that can accommodate the bonding requirements of atoms in a molecule. In ethane (C2H6), the carbon atoms undergo sp3 hybridization, resulting in four equivalent sp3 hybrid orbitals that form sigma bonds with hydrogen atoms and between the carbon atoms. This hybridization leads to a tetrahedral geometry with bond angles of approximately 109.5 degrees.
Recommended video:
Guided course

Hybridization
VSEPR Theory
Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of individual molecules based on the repulsion between electron pairs in the valence shell of the central atom. According to VSEPR, electron pairs will arrange themselves to minimize repulsion, leading to specific molecular shapes. In the case of ethane with sp3 hybridization, the tetrahedral arrangement minimizes repulsion, resulting in a more stable structure compared to the trigonal planar arrangement of sp2 hybridized carbons.
Recommended video:
Guided course

Molecular Shapes and VSEPR
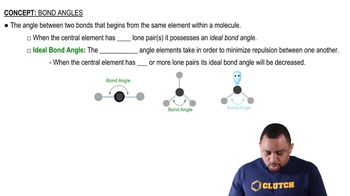
Bond Angles
Bond angles are the angles formed between two adjacent bonds at an atom in a molecule. In ethane, the sp3 hybridized carbon atoms create bond angles of about 109.5 degrees due to their tetrahedral arrangement. In contrast, if the carbon atoms were sp2 hybridized, the bond angles would be approximately 120 degrees, leading to a less stable configuration in the context of ethane's actual structure, which favors the sp3 hybridization.
Recommended video:
Guided course
Bond Angles
Related Practice
