Chemical Bonding
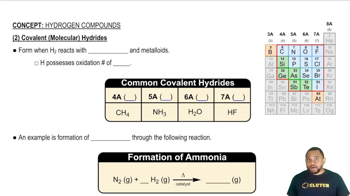
Chemical bonding refers to the forces that hold atoms together in a compound. There are two primary types of bonds: ionic and covalent. Ionic bonds form when electrons are transferred from one atom to another, typically between metals and nonmetals, resulting in charged ions. Covalent bonds, on the other hand, involve the sharing of electrons between atoms, usually between nonmetals.

 Verified step by step guidance
Verified step by step guidance