a. Why is the SO3 molecule trigonal planar but the SO32– ion is trigonal pyramidal?

Write a balanced net ionic equation for the reaction of the amphoteric oxide Ga2O3 with:
a. Aqueous sulfuric acid
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Amphoteric Oxides
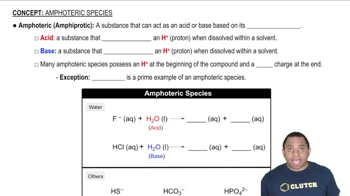
Net Ionic Equations

Acid-Base Reactions

An important physiological reaction of nitric oxide (NO) is its interaction with the superoxide ion (O2–) to form the peroxynitrite ion (ONOO–).
a. Write electron-dot structures for NO, O2–, and ONOO–, and predict the O–N–O bond angle in ONOO–.
Which compound in each of the following pairs is more covalent?
(a) PCl3 or AlF3
Which element in each of the following pairs has more nonmetallic character?
(a) Se or Te
Write a balanced net ionic equation for the reaction of the amphoteric oxide ZnO with:
a. Hydrochloric acid
In the following pictures of binary hydrides, ivory spheres
represent H atoms or ions, and burgundy spheres represent
atoms or ions of the other element.
(1)
(2)
(3)
(4)
(a) Identify each binary hydride as ionic, covalent, or interstitial.
(b) What is the oxidation state of hydrogen in compounds (1), (2), and (3)? What is the oxidation state of the other
element?
