Textbook Question
Arrange the following oxides in order of increasing basic character: Al2O3, Cs2O, K2O, N2O5.

 Verified step by step guidance
Verified step by step guidance
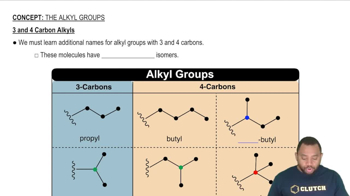

Arrange the following oxides in order of increasing basic character: Al2O3, Cs2O, K2O, N2O5.
Suggest a plausible structure for the silicate anion in the mineral thortveitite, Sc2Si2O7.
Describe the structure of diborane (B2H6) and explain why the bridging B–H bonds are longer than the terminal B–H bonds.
Using the shorthand notation of Figure 22.9, draw the structure of the silicate anion in:
(a) K4SiO4 (b) Ag10Si4O13
What is the relationship between the charge on the anion and the number of terminal O atoms?
What is the hybridization and geometry around carbon atoms in graphene? Explain why graphene is an excellent conductor of electricity.
List three ways in which the properties of boron differ from those of the other group 3A elements.