Textbook Question
Identify the group 3A element that best fits each of the following descriptions.
(c) Is a semiconductor

 Verified step by step guidance
Verified step by step guidance
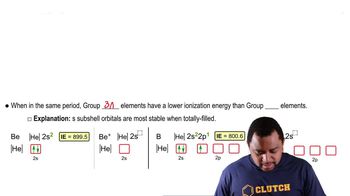

Identify the group 3A element that best fits each of the following descriptions.
(c) Is a semiconductor
Identify the group 3A element that best fits each of the following descriptions.
(a) Has an unusually low melting point
What is the most common oxidation state for each of the
group 3A elements?
(a) Describe what is meant by an electron-deficient molecule.
(b) Describe what is meant by a three-center, two-electron bond.
Suggest a structure for the mixed aluminum–boron hydride AlBH6.