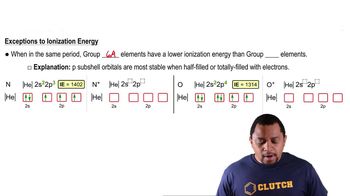
Group 5A Elements
Group 5A elements, also known as Group 15 in the periodic table, include nitrogen, phosphorus, arsenic, antimony, and bismuth. These elements have five valence electrons, which influence their chemical behavior and oxidation states. Understanding their properties and common oxidation states is crucial for identifying which element fits specific descriptions.

 Verified step by step guidance
Verified step by step guidance