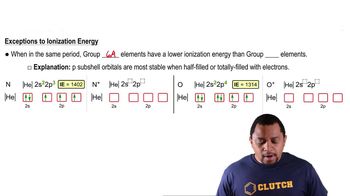
Group 5A Elements
Group 5A elements, also known as the nitrogen group, include nitrogen, phosphorus, arsenic, antimony, and bismuth. These elements exhibit varying oxidation states and can form a range of compounds, such as acids and oxides. Recognizing their common oxidation states and typical compounds is crucial for predicting chemical behavior and reactivity.

 Verified step by step guidance
Verified step by step guidance