Textbook Question
Explain why [CoCl4]2- (blue) and [Co(H2O)6]2+ (pink) have different colors. Which complex has its absorption bands at longer wavelengths?

 Verified step by step guidance
Verified step by step guidance

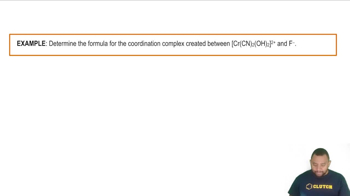
Explain why [CoCl4]2- (blue) and [Co(H2O)6]2+ (pink) have different colors. Which complex has its absorption bands at longer wavelengths?
Draw all possible diastereoisomers of [Cr(C2O4)2(H2O)2]-. Which can exist as a pair of enantiomers?
Draw a crystal field energy-level diagram for a square planar complex, and explain why square planar geometry is especially common for d8 complexes.
Predict the number of unpaired electrons for each of the following.
(c) Zn2+
(d) Cr3+
What is the general trend in standard potentials for the oxidation of first-series transition metals from Sc to Zn? What is the reason for the trend?
What is the crystal field energy level diagram for the complex [Fe(NH3)6]3+?
(a)
(b)
(c)
(d)