Textbook Question
Which of the following transition metals have more than one oxidation state?
(a) Ti
(b) V
(c) Cr
(d) Zn

 Verified step by step guidance
Verified step by step guidance

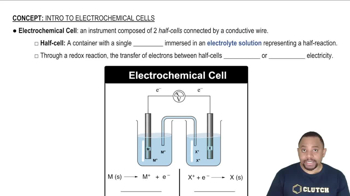
Which of the following transition metals have more than one oxidation state?
(a) Ti
(b) V
(c) Cr
(d) Zn
The highest oxidation state for the early transition metals Sc, Ti, V, Cr, and Mn is the periodic group number. The highest oxidation state for the later transition elements Fe, Co, and Ni is less than the periodic group number. Explain.
Do you expect a compound with vanadium in the +2 oxidation state to be an oxidizing or a reducing agent? Explain.
Will a compound that contains a Fe6+ ion be an oxidizing agent or a reducing agent? Explain.