For each of the following, (i) give the systematic name of the compound and specify the oxidation state of the transition metal, (ii) draw a crystal field energy-level diagram and assign the d electrons to orbitals, (iii) indicate whether the complex is high-spin or low-spin (for d4 - d7 complexes), and (iv) specify the number of unpaired electrons. (d) K4[Os(CN)6]

The drug Nipride, Na2[Fe(CN)5NO], is an inorganic complex used as a source of NO to lower blood pressure during surgery. (a) The nitrosyl ligand in this complex is believed to be NO+ rather than neutral NO. What is the oxidation state of iron, and what is the systematic name for Na2[Fe(CN)5NO]? (b) Draw a crystal field energy-level diagram for [Fe(CN)5NO]2-, assign the electrons to orbitals, and predict the number of unpaired electrons.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Oxidation State

Coordination Complex Nomenclature
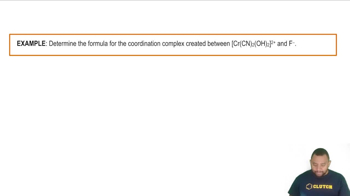
Crystal Field Theory

For each of the following, (i) give the systematic name of the compound and specify the oxidation state of the transition metal, (ii) draw a crystal field energy-level diagram and assign the d electrons to orbitals, (iii) indicate whether the complex is high-spin or low-spin (for d4 - d7 complexes), and (iv) specify the number of unpaired electrons.
(e) [Pt(NH3)4](ClO4)2
For each of the following, (i) give the systematic name of the compound and specify the oxidation state of the transition metal, (ii) draw a crystal field energy-level diagram and assign the d electrons to orbitals, (iii) indicate whether the complex is high-spin or low-spin (for d4 - d7 complexes), and (iv) specify the number of unpaired electrons.
(f) Na2[Fe(CO)4]
Give a valence bond description of the bonding in each of the following complexes. Include orbital diagrams for the free metal ion and the metal ion in the complex. Indicate which hybrid orbitals the metal ion uses for bonding, and specify the number of unpaired electrons.
(a) [Ti(H2O)6]3+
Give a valence bond description of the bonding in each of the following complexes. Include orbital diagrams for the free metal ion and the metal ion in the complex. Indicate which hybrid orbitals the metal ion uses for bonding, and specify the number of unpaired electrons.
(c) [Fe(CN)6]3- (low-spin)
